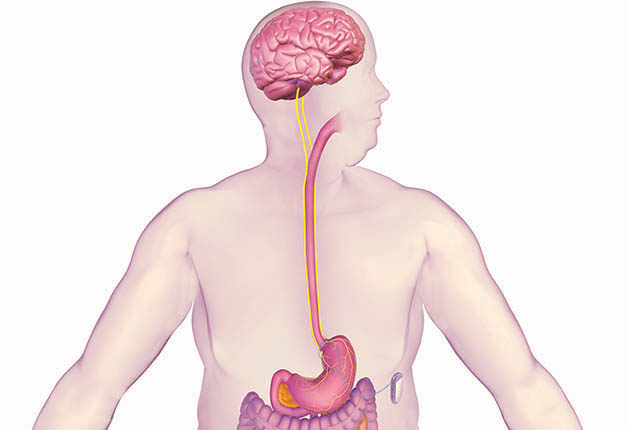
Photo courtesy of EnteroMedics
Details about how the Maestro Rechargeable System actually works are unclear, but the FDA found the evidence to be favorable enough for approval. In a clinical trial that included 233 patients with a BMI of 35 or greater, of the 157 patients who received active Maestro devices, 52.5% lost at least 20% of their excess weight and 38% of them lost at least 25% of their excess weight. Overall, the experimental group lost 8.5% more of their excess weight than the control group after 12 months.
After evaluating the 18-month data, which showed sustained weight loss, the FDA Advisory Committee agreed that the benefits of the device outweighed the risks, which include nausea, pain at the site where the device is placed, vomiting, as well as surgical complications.
But the FDA wants more information. Though the Maestro is approved for treatment in adults over the age of 18 who have not been successful in losing weight with a program, and who have a body mass index of 35 to 45 with at least one other obesity-related condition (like type 2 diabetes), the FDA is requiring the company that makes the device to conduct a five-year post-approval study. It will follow 100 patients to collect additional safety and effectiveness data including weight loss, adverse events, surgical revisions and explants and changes in obesity-related conditions. "How this system will fare in terms of long-term treatment effectiveness remains to be seen," Ochner says.




